ADEPT®

The Burden of Postoperative Adhesions
20-40% of infertility cases in women are caused by adhesions1
#1 cause of secondary infertility1
34.5 % of patients re-admitted due to adhesions
After open gynaecological surgery, patients were admitted almost twice (1.9 times) for a problem potentially related to adhesions or for further surgery potentially complicated by adhesions over a 10-year period2
Results based on Clinical Studies
Simple and Effective
Simple and Effective
Direct and Fast-Acting Application
As a liquid, ADEPT® is administered directly and rapidly to the site through a laparoscopic port during surgery3
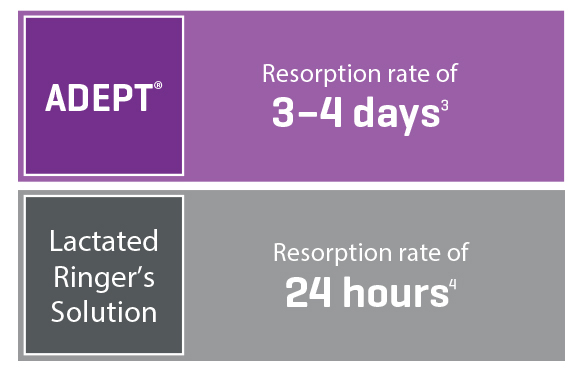
Broad Coverage and Optimised Resorption
Ingredient icodextrin slows the resorption time, whereas regular fluids would be resorbed much faster5
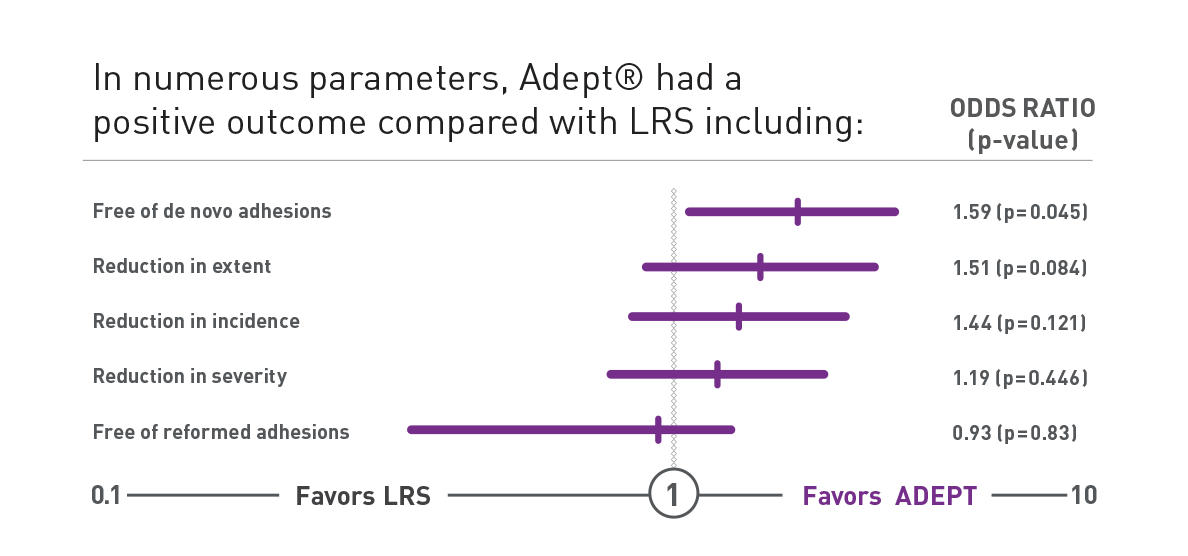
PAMELA Study
Double-blind randomised controlled trial comparing ADEPT® (n=203) and Lactated Ringer's Solution (n=199) during laparoscopic gynaecological surgery6

Ariel Registry
Pan European registry of ADEPT® use in 2,069 gynaecologic patients7
Rated most frequently by surgeons as ‘good’ or ‘excellent’ in laparoscopy cohorts
Can be used without requiring significant changes in surgical practice
Considered easy to use by participating surgeons
Overall satisfaction with ADEPT® as good or excellent in >90% of procedures
Baxter takes safety of our products and patients very seriously. If you would like to report an adverse event with Baxter drugs (e.g. Tisseel, Artiss, …), you can contact Baxter directly: [email protected], or you can report it via Netherlands Pharmacovigilance Center Lareb’s website: www.lareb.nl Any medical device product quality complaints (including medical device adverse incidents) relating to Baxter products should be sent to EMEA SHS Complaints Intake [email protected]