SEPRAFILM

Adhesions are not preventable by surgical technique alone
Adhesions develop routinely following both open and laparoscopic abdominal surgery, and have been reported at second-look surgery to occur in up to 93% of patients (n=210) following laparotomy2.
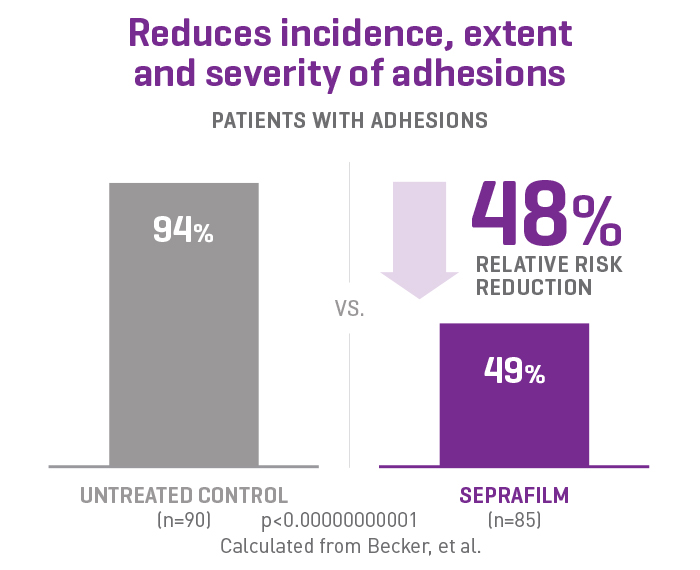
SEPRAFILM efficacy in abdominal surgery
In a randomised, prospective, double-blinded, multicenter clinical study involving 183 patients [175 evaluable] with ulcerative colitis and familiar polyposis undergoing 2-stage intestinal resection, more than half of the patients treated with SEPRAFILM were adhesion-free at 12 weeks compared to 6% of untreated patients2.
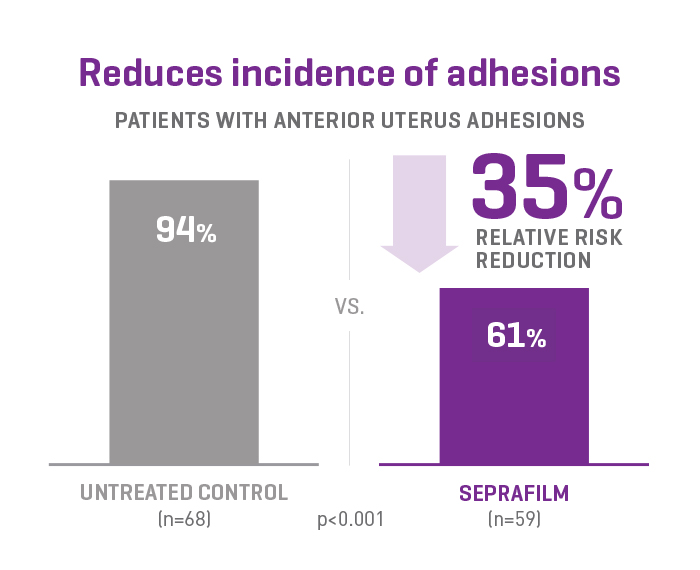
SEPRAFILM efficacy in pelvic surgery
In a prospective, randomised, blinded, multicenter clinical study involving 127 patients undergoing gynecologic surgery, SEPRAFILM reduced the mean number of sites adherent to the anterior uterine surface following myomectomy compared with untreated patients. SEPRAFILM also significantly reduced the extent and severity of adhesions in patients undergoing uterine myomectomy compared with untreated patients3.
Additional Product Benefits
Protection When It Matters Most
Separates tissues for up to 7 days - the critical tissue healing period1,2
A Proven Legacy
More than 4 million patients have received SEPRAFILM in clinical use worldwide. Robust clinical data (n=2,133) across five clinical studies have demonstrated the safety and efficacy of SEPRAFILM Adhesion Barrier. 2-6
Bioresorbable and Synthetic
SEPRAFILM is composed of sodium hyaluronate and carboxymethylcellulose (HA/CMC)
Baxter takes safety of our products and patients very seriously. If you would like to report an adverse event with Baxter drugs (e.g. Tisseel, Artiss, …), you can contact Baxter directly: [email protected], or you can report it via Netherlands Pharmacovigilance Center Lareb’s website: www.lareb.nl Any medical device product quality complaints (including medical device adverse incidents) relating to Baxter products should be sent to EMEA SHS Complaints Intake [email protected]